
Innovations and future tech spotted at this year's MEDICA event
November 2023
Our Healthcare and Life Sciences team attended MEDICA 2023 this month, one of the largest medical trade fairs in the world, showcasing a wide range of innovative products and services. We discuss some of the highlights.
In this article, we summarise six main themes from this event that are driven by innovation and regulation in the sector.
.jpg?width=737&height=492&name=shutterstock_1847981734%20(4).jpg)
Custom PCR modules
A trend emerging from the busy PCR machine market is that more and more kit manufacturers are considering breaking into the space by developing their own custom PCR modules.
No longer content to just supply the consumables and software packages, the deep knowledge of their unique kit chemistries allows for the customisation of modules and machines towards fast, real-time PCR for point-of-care (POC) applications. With total cycle times on the order of 5 minutes, this seems by far the biggest growth segment holding the promise of high throughput, multi-factor diagnostics.
Wearables innovation
Wearables are a broad catch-all for everything from ‘health watches’ that track sleep cycles and pulse rates, to more targeted localised devices analysing sweat composition, nerve signals or blood oxygen concentration.
.webp?width=758&height=427&name=shutterstock_757101553%20(2).webp)
All of these devices require power to run and an interesting innovation that caught our eye was the development of Bioenzymatic Fuel Cells. Composed of paper and organic chemistry, these cells can apparently provide about 11mAh per cm2 and are disposable, recyclable, environmentally friendly and economically viable.
With sustainability a pervasive concern across the industry and single-use device disposal, such as vapes, a growing concern, we will follow development and adoption of these novel power sources closely.
Microfluidic device manufacture
Fast and targeted diagnostics, be it for next generation sequencing, single-cell analytics or other applications within the exploding field of Omics often require precise dosing and positioning, which microfluidic devices can enable.
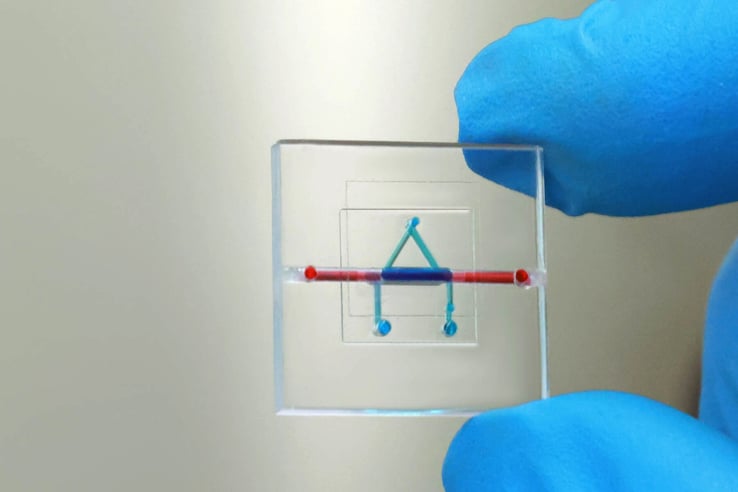
While prototyping cost and timescales have reduced, scale up of microfluidic consumables to large quantities can be a high barrier to market.
Skilled suppliers have recognised this. Through material and process innovation such as continuous laminated plastic rolls, design for manufacture consultation, and forming diverse end-to-end supplier networks, they have given new ventures a much more viable route to market.
Pain management
The worrying increase of opioid use and addiction in chronic and post-operative pain management has sparked alternative approaches that do not rely on drugs.
One such device we were fascinated by targets the auricular vagus nerve through electric stimulation within the ear. The small device has 3 electrodes that are inserted by a medical professional and supplied by a control and battery unit attached behind the ear.
.webp?width=750&height=501&name=shutterstock_1442092214%20(1).webp)
A course for chronic pain typically lasts 6 weeks with weekly device replacement and the majority of studies within a systematic review show “significant and clinically relevant reduction in pain” and “significant reduction in the use of analgesics or opiates.”
This is a wonderful confluence of medical understanding and targeted device engineering that provides better quality of life for patients, especially for chronic pain management, without the potential complications of long-term medication use.
Sustainability
This was a topic that came up across all areas of the show. Several companies exhibited innovative new products designed with sustainability at their core.
Lateral flow tests are one product starting to go plastic-free, with various home-use pregnancy tests now available that are fully or mostly paper-based.
Additionally, companies engaged in insightful discussions about sustainability projects in their R&D pipelines. This dual emphasis on product innovation and sustainable practices underscores a promising evolution in the industry's approach to meeting healthcare needs while addressing broader global concerns.
Regulatory transition
More than six years since their publication, the EU regulatory transition for Medical Devices (MDR) and In Vitro Diagnostics (IVDR) is still causing delays in bringing some new products to market.
The changes towards better traceability, requiring post-market clinical follow-up, surveillance and vigilance have also slowed down the ability to make some changes and improvements to existing products.
This has mostly been due to Notified Body availability. Hopefully the situation is improving, such that the changes intended to provide greater public health protection and patient safety do not jeopardize crucial innovation in efficacy and usability for better treatment outcomes.
In summary, we found several exciting new frontiers of innovation at MEDICA 2023. Be it for individual, highly personal wearables and medical devices or advanced, fast diagnostics for POC applications, drug discovery or gene therapies, all against a backdrop of a drive towards greater sustainability, and regulatory compliance.

Our Healthcare and Life Sciences team at MEDICA (left to right): Matthias Ediger, Sarah Knight, Claire Lebouteiller, Marta Uncio Ribera, Michaela Hume and Carl Pullen
With the goal to enhance people’s quality of life at the heart, the 42T Healthcare and Life Sciences team is excited to continue to contribute to these efforts with our clients and partners. We’re ideally placed to help with the associated product and process development challenges in the timely and efficient manner they require.
To find out about other trends, sign up to our Healthcare and other newsletters.
If you would like to find out more
Contact our Head of Life Science, Matthias Ediger

matthias.ediger@42T.com | +44 (0)1480 309461 | LinkedIn: Matthias
Share this article:
Related Articles

Healthcare & Life Sciences
The future of home healthcare: trends and innovation

Healthcare & Life Sciences
Right to repair: reshaping the medical equipment industry

Healthcare & Life Sciences
The hidden risks with smart injectors - is high-tech sacrificing usability?

What will you ask us today?
We believe in asking the right questions to drive innovation; when we know the right questions, we generate the ideas to answer them.

